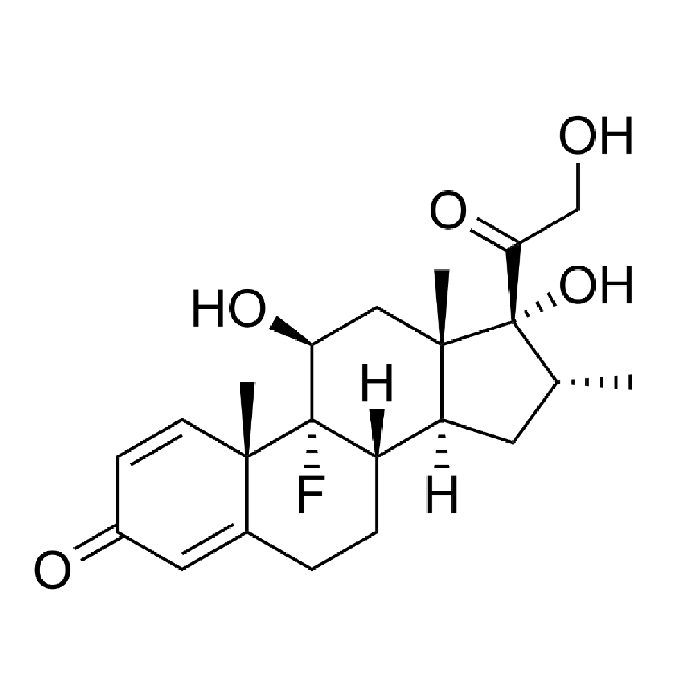
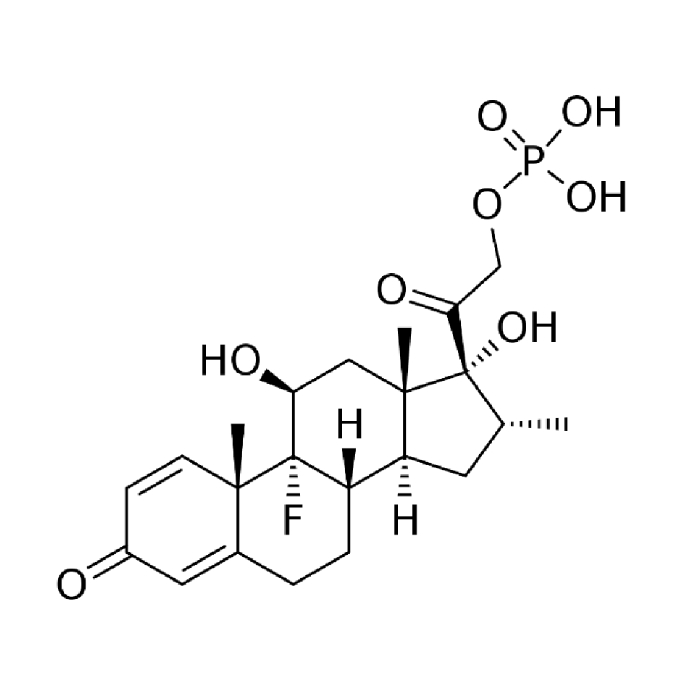
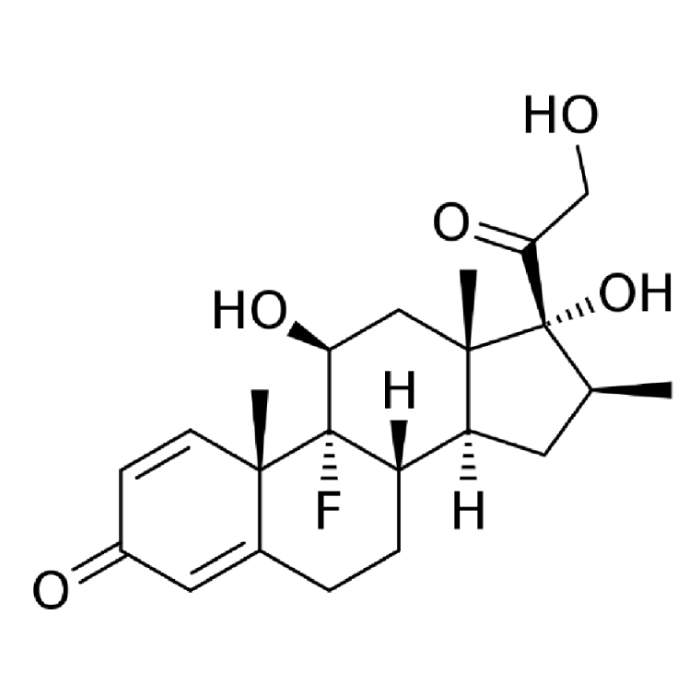
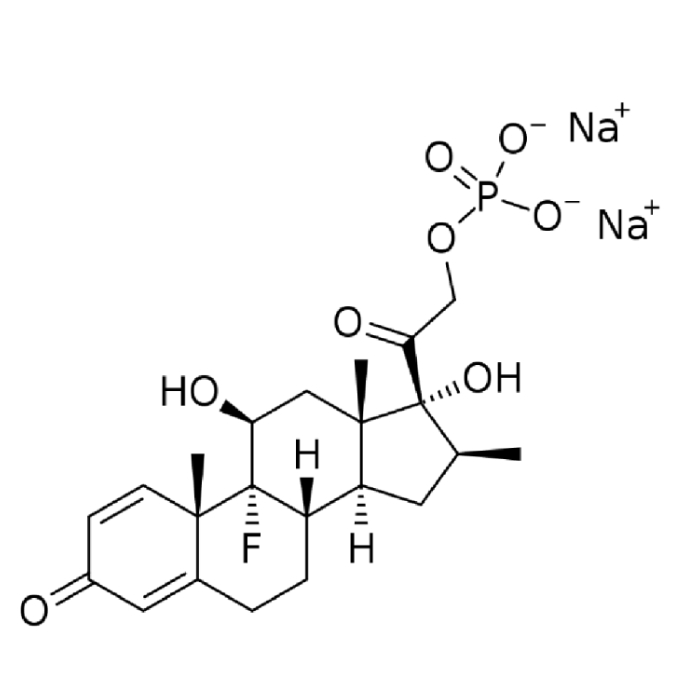
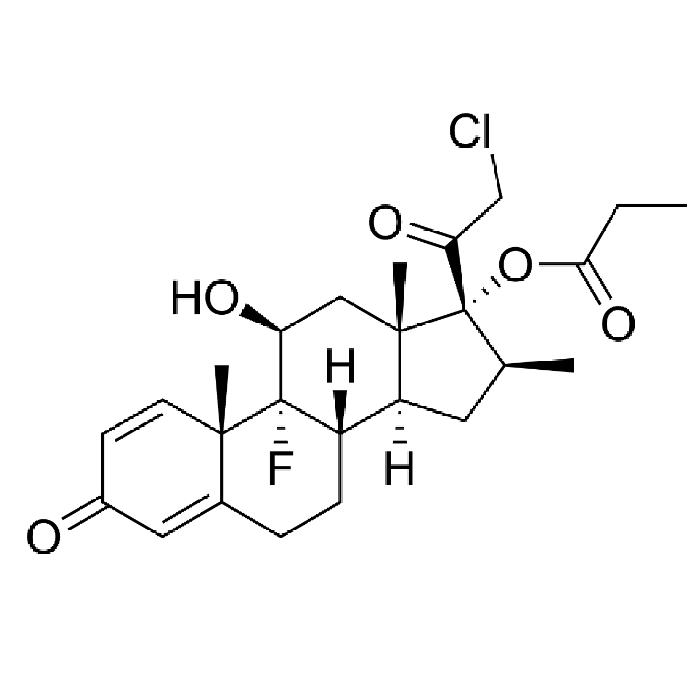
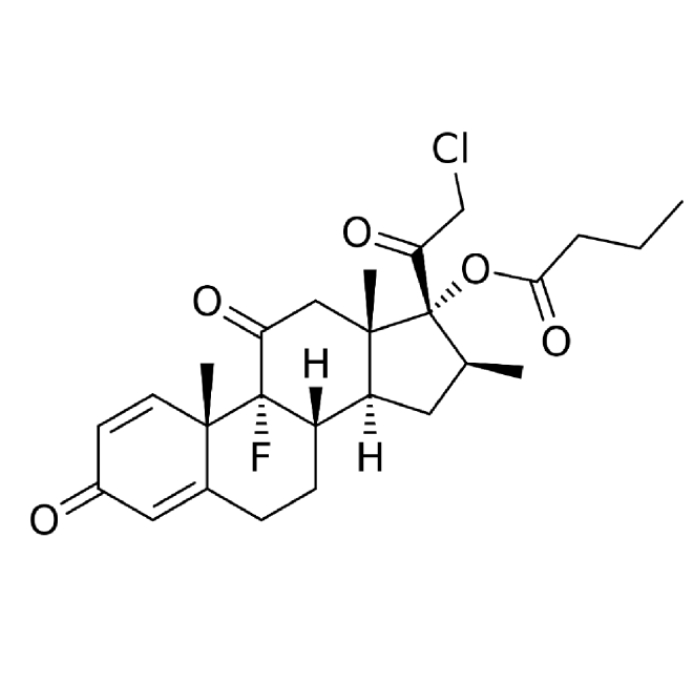
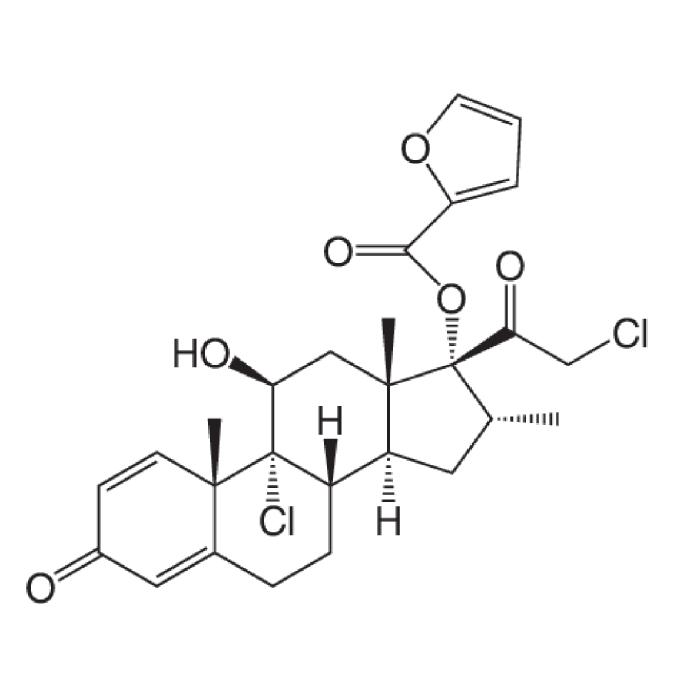
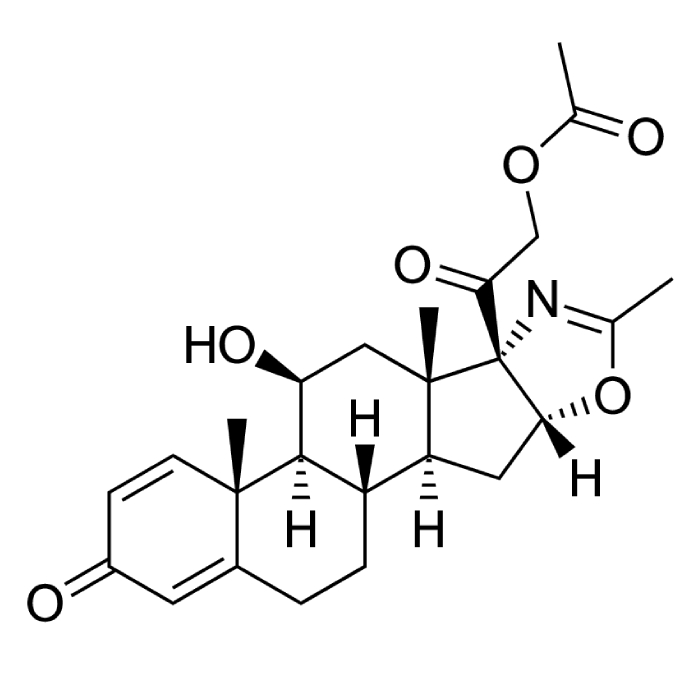
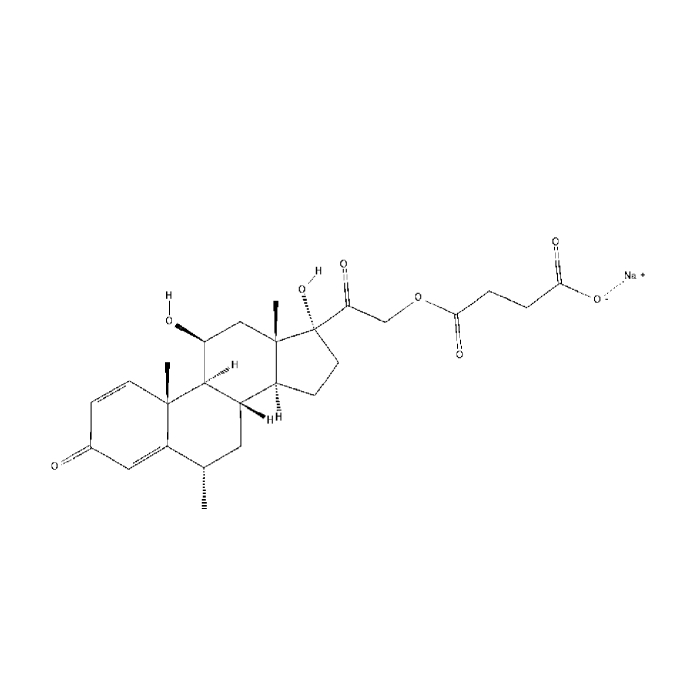
In year 2016, the above said company after producing Hormones, steroids and General API over long 4 years at Chhatral (Gandhinagar) city site felt badly the need to upgrade itself as per new GMP & off-course to cater to ever increasing domestic as well as international demand.
CONTACT US
- (+91) 98 9857 3384
- sales@cropel.in
- Plot No.3203, Phase III, G.I.D.C. Chhatral-382729Ta. -Kalol, Dis.-Gandhinagar, Gujarat, India